The electron configuration of an atom is
a form of notation which shows how the electrons are distributed among the various atomic
orbitals and energy levels. The format consists of a series of numbers, letters and
superscripts as shown below:
1s2
Here we see the
electron configuration for the element helium. This electron configuration provides
us with the following information:
The large number "1" refers to the
principle quantum number "n" which stands for the energy level. It tells
us that the electrons of helium occupy the first energy level of the atom.
The letter "s" stands for the angular
momentum quantum number "l". It tells us that the two electrons of the
helium electron occupy an "s" or spherical orbital.
The exponent "2" refers to the total
number of electrons in that orbital or sub-shell. In this case, we know that there
are two electrons in the spherical orbital at the first energy level.
Before we continue, we must
review some information that you will need to fill in electron configurations correctly.
I. Principle Quantum Number (n) and
Sublevels
The number of sublevels that an energy level can
contain is equal to the principle quantum number of that level. So, for example, the
second energy level would have two sublevels, and the third energy level would have three
sublevels. The first sublevel is called an s sublevel. The second sublevel is
called a p sublevel. The third sublevel is called a d sublevel and the fourth
sublevel is called an f sublevel. Although energy levels that are higher than 4
would contain additional sublevels, these sublevels have not been named because no known
atom in its ground state would have electrons that occupy them.
II. Sublevels and Orbitals
An orbital is a space that can be occupied by up to
two electrons. Each type of sublevel holds a different number or orbitals, and
therefore, a different number of electrons. s sublevels have one orbital, which can
hold up to two electrons. p sublevels have three orbitals, each of which can hold 2
electrons, for a total of 6. d sublevels have 5 orbitals, for a possible total of 10
electrons. f sublevels, with 7 orbitals, can hold up to 14 electrons. The
information about the sublevels is summarized in the table below:
Table
3-6a - Orbital and Electron Capacity for the Four Named Sublevels |
| Sublevel |
# of orbitals |
Maximum number of electrons |
| s |
1 |
2 |
| p |
3 |
6 |
| d |
5 |
10 |
| f |
7 |
14 |
III. Total Number of
Orbital and Electrons per Energy Level
An easy way to calculate the number of orbitals
found in an energy level is to use the formula n2. For example, the third
energy level (n=3) has a total of 32, or nine orbitals. This makes sense
because we know that the third energy level would have 3 sublevels; an s sublevel with one
orbital, a p sublevel with 3 orbitals and a d sublevel with 5 orbitals. 1 + 3 + 5 =
9, so the formula n2 works!
IV. Total Number of Electrons per
Energy Level
An easy way to calculate the total number of
electrons that can be held by a given energy level is to use the formula 2n2.
For example, the fourth energy level (n=4) can hold 2(4)2 = 32
electrons. This makes sense because the fourth energy level would have four
sublevels, one of each of the named types. The s sublevel hold 2 electrons, the p
sublevel holds 6 electrons , the d sublevel holds 10 electrons and the f sublevel holds 14
electrons. 2 + 6 + 10 + 14 = 32, so the formula 2n2 works! We can
summarize this information in the table below:
| Table 3-6b
Orbitals and Electron Capacity of the First Four Principle Energy Levels |
| Principle energy level (n) |
Type of sublevel |
Number of orbitals per type |
Number of orbitals per
level(n2) |
Maximum number of electrons
(2n2) |
| 1 |
s |
1 |
1 |
2 |
| 2 |
s |
1 |
4 |
8 |
| p |
3 |
| 3 |
s |
1 |
9 |
18 |
| p |
3 |
| d |
5 |
| 4 |
s |
1 |
16 |
32 |
| p |
3 |
| d |
5 |
| f |
7 |
V. Order of Filling Sublevels with
Electrons
The next thing that you need to recall is the fact
that the energy sublevels are filled in a specific order that is shown by the arrow
diagram seen below:
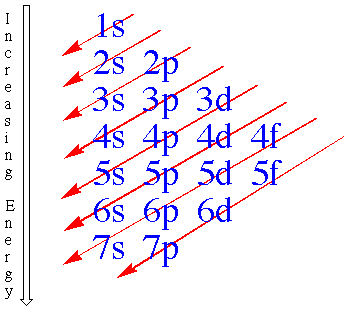
Remember to start at the beginning of each arrow,
and then follow it all of the way to the end, filling in the sublevels that it passes
through. In other words, the order for filling in the sublevels becomes; 1s, 2s, 2p,
3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d,7p.
|
